A Policy and Funding Evaluation Of Human Fetal Tissue Research
This is Issue 2 of the On Science.
Background
The body parts of preborn babies that die through the act of elective abortion are harvested and sold to desiring scientists for experimentation. These acts are heinous, controversial, and have a history of ethical misconduct.[1]
The Trump administration has taken bold and deliberate actions that protect and respect the dignity of human life from conception to natural death. The administration has made changes in federal funding policy for research using human fetal tissue from elective abortions, to be in better alignment with ethical research practices.
Here we evaluate the actions and policy changes made by the Trump administration that have been taken since his term in office and analyze the current standing of federal funds used to support fetal tissue research. First, we give a brief overview of how federal tax dollars are used to fund medical research in the U.S.
Federal Funding of Medical Research
As part of the U.S. Department of Health and Human Services (HHS), the National Institutes of Health (NIH) is the primary agency responsible for funding medical research. Since American taxpayer dollars fund the NIH budget, the American people are the primary benefactors and beneficiaries of advances in scientific research. The NIH invests approximately $41.7 billion annually into biomedical research, of which the majority (>80%) is awarded for extramural research through competitive grants to more than 300,000 researchers at more than 2,500 universities, medical schools, and research institutions throughout the country. A smaller portion of the NIH budget (~10%) supports intramural research conducted in its own NIH laboratories and through contracts with other universities.
Most Americans oppose using tax dollars to pay for a woman’s abortion, let alone having more of their tax dollars used for purchasing and experimenting on aborted baby body parts. Furthermore, the use of aborted fetal tissues in the production of any medicines becomes highly problematic for anyone who must choose between violating their conscience or accepting a treatment tainted with abortion-derived materials.
Administrative Actions and Policies
A timeline of policies enacted by the Trump administration regarding research using human fetal tissue from elective abortions is outlined below.
- September 24, 2018: The Department of Health and Human Services (HHS) terminated a contract between Advanced Bioscience Resources (ABR), Inc. and the Food and Drug Administration that provided human fetal tissue from elective abortions to develop testing protocols. The Department was not sufficiently assured the contract between the FDA and ABR included the appropriate protections applicable to fetal tissue research or met all other procurement requirements. The department initiated an audit of all acquisitions involving human fetal tissue to ensure conformity with procurement and human fetal tissue research laws and regulations.
- December 10, 2018: NIH announced a $20 million funding opportunity for research to develop, demonstrate, and validate experimental models that do not rely on human fetal tissue from elective abortions. This action demonstrated that HHS is committed to providing additional funding to support the development and validation of alternative models.
- June 5, 2019: Results from the HHS audit and review led to the administration’s decision to let a contract with the University of California, San Francisco (UCSF) expire, which involved HIV studies using human immune system (HIS) mice (humanized mice) that were generated with aborted fetal livers and thymuses. HHS discontinued intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. No current extramural research projects (research conducted outside NIH, e.g., at universities, that are funded by NIH grants) would be affected during their currently approved project period. An Ethics Advisory Board would be convened to review future extramural research grant applications that propose to use fetal tissue from elective abortions.
- July 26, 2019: HHS issued a notice (NOT-OD-19-128) requiring a statutory ethics review of extramural proposals submitted on or after September 25, 2019. This affected new extramural research grant applications or current research projects in the competitive renewal process that propose to use fetal tissue from elective abortions and that are recommended for potential funding through NIH’s two-level external scientific review process. NIH implemented requirements in which investigators must provide scientific justification of the use of human fetal tissue (in light of available alternatives) with details regarding procurement and costs, and information about how the applicant/contract offeror will use HFT.
- July 31, 2020: The new NIH Human Fetal Tissue Research Ethics Advisory Board for Fiscal Year 2020 convened to review research involving human fetal tissue proposed in competitive NIH grant and cooperative agreement applications and recommend whether, in light of the ethical considerations, NIH should fund the research project—pursuant to a law passed by Congress. The recommendations will advise the Secretary on whether federal funds should or should not be withheld because of ethical considerations. The advisory board is required to submit its findings no later than August 18, 2020, and it will dissolve 30 days after it does so. In the meantime, any new or competitive renewals that propose to use human fetal tissue from elective abortions that are within a fundable scoring range and R&D contract proposals that have been identified through the Source Selection process as apparently successful offerors, will not receive any federal funds to conduct the proposed studies.
- August 18, 2020: The Board submitted their recommendations to the HHS Secretary. The public report disclosed that the Board discussed a total of 14 research proposals (including both grants and contracts) at the July 31 meeting. Consensus by the Board was not required, and recommendations were determined by a simple majority. The Board was charged to consider only ethical aspects of the human fetal tissue from abortions used in the research proposals. The Board voted to recommend that the Secretary:
- withhold funds for thirteen of the research proposals
- not withhold funds for one of the research proposals.
The Secretary will make any funding decisions based on the recommendations of the Board, as it pertains to components of the proposals that use HFT. The final Report of the Human Fetal Tissue Research Ethics Advisory Board-2020 describes the detailed findings and recommendations to the Secretary. It should be noted that there was 100% consensus from all board members (15 to 0 vote) to withhold funds for two of the research proposals and nearly unanimous consensus (14 to 1) to withhold funds for three research proposals.
These actions taken by the Trump administration over the last two years are important for several reasons. They blocked federal dollars from supporting a subset of fetal tissue research from elective abortions, they ensured that appropriate protections applicable to research involving human fetal tissue from elective abortions are consistent with statutes and regulations governing such research, and they guaranteed the adequacy of procedures and oversight of this research in light of the serious regulatory, moral, and ethical considerations involved. Finally, they supported and accelerated ethical alternatives that do not rely on human fetal tissue from abortions.
Extramural Funding of Fetal Tissue Research
In light of fetal tissue policy changes outlined above, we performed a comprehensive tracking study to get a snapshot of the number of NIH-funded proposals that currently use human fetal tissue from elective abortions. The total dollar amount supporting human fetal tissue research is reported by the Department of Health and Human Services (HHS), NIH Categorical Spending, which estimates funding for various research, condition, and disease categories (RCDC). When reporting categorical spending, NIH does not distinguish between human fetal tissue from miscarriages/stillbirths and elective abortions. Therefore, our search identified human fetal tissue research using both illicit (i.e., elective abortion) and licit means (i.e., natural death, miscarriage).
The total amount of funding of fetal tissue research for fiscal years (FY) 2016-2021 is summarized in Table I below. Fiscal years that precede the current funding cycle are in actual dollar amounts, whereas current and future amounts are estimated spending. According to these data, approximately 0.3% of the total NIH budget funds research using human fetal tissue. The vast majority (>99%) does not.
| TABLE I. NIH Funding of Human Fetal Tissue Research | ||||||
| Research/Disease Area
|
FY 2016 Actual | FY 2017 Actual | FY 2018 Actual | FY 2019 Estimated | FY 2020 Estimated | FY 2021 Estimated |
| Human Fetal Tissue
(Dollars in millions, rounded) |
$103 | $98 | $115 | $109 | $116 | $107 |
| Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC)
Table published: February 24, 2020 Accessed 11 May 2020, via search on “human fetal tissue” at: http://report.nih.gov/categorical_spending.aspx |
||||||
Fetal Tissue Projects Funded in FY 2019
Each extramural human fetal tissue project funded in FY 2019 (any grant approved for funding during the period of October 1, 2018-September 30, 2019) was then analyzed. Data was exported from the search described above (in red) and each project was individually queried and analyzed using the Federal Research Portfolio Online Reporting Tools (RePORT), a searchable database of NIH-funded research projects. There are many different types of NIH Research Project Grant Programs, with the most common type being the R01 generally awarded for 3-5 years. Therefore, depending on the type of project that is funded, and when funding started, the fetal tissue grants analyzed for FY 2019 are in various stages of the funding cycle (i.e., different start and end dates).
Project information for each grant award was collected. If an investigator obtained multiple awards from different funding institutes (e.g., NIAID, NINDS, NIDDK, etc.) for the same project number, the data were condensed and the cumulative award amount was reported. Program projects (collaborations between different investigators, sometimes at different organizations) that were awarded funding for fetal tissue research were included in the analysis; each subproject of the program project was counted separately. Intramural projects at NIH facilities that showed up in the search were excluded from analysis because intramural projects are forbidden to use fetal tissue from abortions, as described above, because of the HHS decision on June 5, 2019.
A summary of the total number of investigators using fetal tissue, the total number of fetal tissue projects, and the project end dates for all projects funded in FY 2019 (at the time of analysis) is shown in Table II. A total of 135 investigators are project leaders (principal investigators) for 144 projects that use human fetal tissue (some investigators receive funding for multiple fetal tissue projects). A total of 35 projects had a project end date in 2020, meaning they should be up for competitive renewal. However, after our analysis was complete, a subset of projects expired in 2020 and their project end date changed to 2021, suggesting that they requested a one-year No-Cost Extension and gained one more year to conduct their studies. Therefore, the actual number of extramural fetal tissue projects ending in 2020 is less, and those for project end year 2021 are likely more.
| TABLE II. FY 2019 Extramural Human Fetal Tissue Projects | |||||||
| Principal Investigators | Total Projects | Project End 2019* | Project End 2020** | Project End 2021 | Project End 2022 | Project End 2023 | Project End 2024 |
| 135 | 144 | 2 | 35 | 52 | 21 | 23 | 11 |
| Data downloaded from https://Report.nih.gov on April 7, 2020. Analysis end date: May 1, 2020
Research/Disease Area: Human Fetal Tissue Fiscal Year Spending Category: FY 2019 *Excludes UCSF contract cancelled June 5, 2019 **After analysis was complete, a subset of projects expired and the end date changed to 2021 |
|||||||
Human Fetal Tissues and Organs Harvested for Research
The majority of projects awarded in FY 2019 (over 50%) investigate some aspect of HIV/AIDS and many use the BLT (bone marrow, liver, thymus) humanized mouse model. These studies require an abortion clinic to harvest human fetal livers and thymuses from ongoing abortions, which are then sold to desiring investigators. Other projects include basic science research to study Zika infection, macular degeneration, infertility, fetal development, and brain disorders. These projects use various fetal organs including but not limited to: eyes, brains, urinary tract tissues, intestines, hearts, gonads, livers and thymuses. Some examples of harvested tissue descriptions excerpted directly from research project applications are given below in Table III. To the best of our knowledge, none of the FY 2019 projects fund human transplant studies.
| TABLE III. Quotes from Selected Human Fetal Tissue Project Description |
| “We will label green and red cone cells in human and fetal eyes using an RNA in situ hybridization technique that successfully distinguishes green and red opsin expression…” |
| “This proposal seeks to advance knowledge of human cerebellar development and malformations using human fetal samples and mouse models…We will conduct the first in-depth analysis of normal human fetal cerebellar development from 4-23 Gestational Weeks.” |
| “…research will focus on comparisons between the rat fetal testis and both mouse and human fetal testis models…” |
| “…secreted placental proteins from these experiments will then be placed on 3D fetal gonads (+/- phthalates, +/- placental proteins), matched by sex and gestational age.” |
| “This application seeks to develop the resource beyond the core fundamental goal of the resource aka the systematic collection, staging, identification and processing of fetal specimens for distribution of tissues and derivatives to scientific recipients.” |
| “The vaccine approach will then be tested in vivo using the bone marrow, liver, thymus (BLT)-humanized mouse model.” |
Extramural Fetal Tissue Funding for FY 2019 by Organization
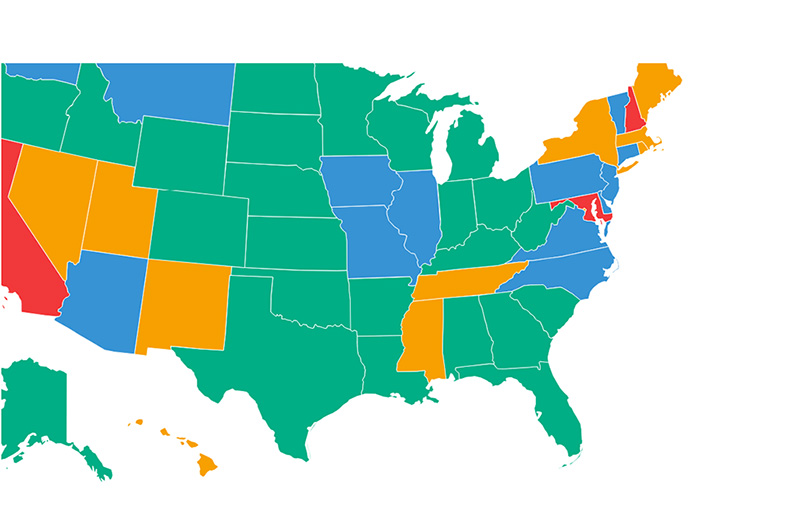
A summary of FY 2019 fetal tissue funding by awardee organization is in Table IV. Sixty-eight organizations in 25 states, plus the District of Columbia, obtained extramural funding in FY 2019 for fetal tissue projects. For 2019, the University of North Carolina at Chapel Hill is the number one awardee of projects that use fetal tissue, with close to $10 million. Two additional fetal tissue projects were awarded to organizations outside the U.S., in South Africa and Spain.
The calculated total amount of funding for the entirety of each fetal tissue project at each organization was determined (data not shown). In one example, the University of Washington was the awardee organization for a total of seven projects that use human fetal tissue in FY 2019. One of these projects entitled “Laboratory of Developmental Biology” funds the Birth Defects Research Laboratory, a fetal tissue repository that routinely provides human fetal tissues from elective abortions to other scientists in the U.S. NIH awarded this lab $794,881 in FY 2019, but the project has been funded for over 50 years, so the total NIH funding amount reported is $13.8 million dollars. The estimated total funding for these FY 2019 fetal tissue projects alone has a history exceeding $500 million.
Note that each grant is typically composed of two or more specific aims and that it is possible that not all aims use fetal tissue to achieve their purpose. Therefore, the total funds used to sponsor fetal tissue research may be something less than the total dollars reported.
| TABLE IV. Fetal Tissue Funding for FY 2019 by Organization and State | ||||
| Organization Name | State | Country | # FY 2019 Projects | FY 2019 Funding |
| CALIMMUNE, INC. | AZ | USA | 1 | $175,263 |
| BECKMAN RESEARCH INSTITUTE/CITY OF HOPE | CA | USA | 1 | $512,462 |
| CALIFORNIA INSTITUTE OF TECHNOLOGY | CA | USA | 4 | $2,423,448 |
| CEDARS-SINAI MEDICAL CENTER | CA | USA | 1 | $550,625 |
| CHILDREN’S HOSPITAL OF LOS ANGELES | CA | USA | 1 | $416,250 |
| NORTHERN CALIFORNIA INSTITUTE/RES/EDU | CA | USA | 1 | $192,612 |
| OAK CREST INSTITUTE OF SCIENCE | CA | USA | 1 | $838,173 |
| SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE | CA | USA | 1 | $664,048 |
| SCRIPPS RESEARCH INSTITUTE | CA | USA | 2 | $1,007,967 |
| STANFORD UNIVERSITY | CA | USA | 2 | $732,775 |
| UNIVERSITY OF CALIFORNIA AT DAVIS | CA | USA | 1 | $206,553 |
| UNIVERSITY OF CALIFORNIA LOS ANGELES** | CA | USA | 12 | $4,753,730 |
| UNIVERSITY OF CALIFORNIA, SAN DIEGO | CA | USA | 1 | $598,306 |
| UNIVERSITY OF CALIFORNIA, SAN FRANCISCO | CA | USA | 5 | $791,287 |
| UNIVERSITY OF SOUTHERN CALIFORNIA | CA | USA | 2 | $821,116 |
| COLORADO STATE UNIVERSITY | CO | USA | 2 | $1,161,459 |
| UNIVERSITY OF CONNECTICUT SCH OF MED/DNT | CT | USA | 1 | $389,500 |
| YALE UNIVERSITY | CT | USA | 3 | $1,195,010 |
| CHILDREN’S RESEARCH INSTITUTE | DC | USA | 1 | $344,888 |
| UNIVERSITY OF FLORIDA | FL | USA | 1 | $721,471 |
| SCRIPPS FLORIDA | FL | USA | 1 | $750,426 |
| EMORY UNIVERSITY | GA | USA | 2 | $1,705,608 |
| UNIVERSITY OF IOWA | IA | USA | 1 | $389,825 |
| BOSTON CHILDREN’S HOSPITAL | MA | USA | 3 | $1,316,237 |
| BOSTON UNIVERSITY MEDICAL CAMPUS | MA | USA | 1 | $401,724 |
| BRIGHAM AND WOMEN’S HOSPITAL | MA | USA | 3* | $2,113,070 |
| BROAD INSTITUTE, INC. | MA | USA | 1 | $102,330 |
| HARVARD MEDICAL SCHOOL | MA | USA | 1 | $32,668 |
| HARVARD UNIVERSITY | MA | USA | 1 | $200,085 |
| MASSACHUSETTS GENERAL HOSPITAL | MA | USA | 2 | $779,740 |
| UNIV OF MASSACHUSETTS MED SCH WORCESTER | MA | USA | 3 | $2,652,304 |
| UNIVERSITY OF MARYLAND BALTIMORE | MD | USA | 1 | $2,210,340 |
| JOHNS HOPKINS UNIVERSITY | MD | USA | 3 | $1,325,727 |
| JACKSON LABORATORY | ME | USA | 1 | $1,088,630 |
| UNIVERSITY OF MICHIGAN AT ANN ARBOR | MI | USA | 1 | $386,627 |
| WASHINGTON UNIVERSITY | MO | USA | 1 | $249,000 |
| MONTANA STATE UNIVERSITY – BOZEMAN | MT | USA | 1 | $625,098 |
| DUKE UNIVERSITY | NC | USA | 3 | $1,536,582 |
| DARTMOUTH COLLEGE | NH | USA | 1 | $647,344 |
| RBHS-NEW JERSEY MEDICAL SCHOOL | NJ | USA | 3 | $2,008,324 |
| AARON DIAMOND AIDS RESEARCH CENTER | NY | USA | 2 | $1,499,415 |
| COLUMBIA UNIVERSITY HEALTH SCIENCES | NY | USA | 5 | $1,565,231 |
| ICAHN SCHOOL OF MEDICINE AT MOUNT SINAI | NY | USA | 1 | $808,293 |
| NEW YORK UNIVERSITY SCHOOL OF MEDICINE | NY | USA | 1 | $506,210 |
| ROCKEFELLER UNIVERSITY | NY | USA | 1 | $939,659 |
| SLOAN-KETTERING INST CAN RESEARCH | NY | USA | 1 | $592,082 |
| STATE UNIVERSITY OF NEW YORK AT BUFFALO | NY | USA | 1 | $348,906 |
| SUNY DOWNSTATE MEDICAL CENTER | NY | USA | 1 | $298,567 |
| UNIVERSITY OF ROCHESTER | NY | USA | 1 | $199,867 |
| WEILL MEDICAL COLL OF CORNELL UNIV | NY | USA | 1 | $748,496 |
| OREGON HEALTH & SCIENCE UNIVERSITY | OR | USA | 2 | $563,924 |
| TEMPLE UNIV OF THE COMMONWEALTH | PA | USA | 1 | $373,362 |
| UNIVERSITY OF PENNSYLVANIA | PA | USA | 8 | $3,143,312 |
| UNIVERSITY OF PITTSBURGH AT PITTSBURGH | PA | USA | 4 | $2,239,726 |
| WISTAR INSTITUTE | PA | USA | 2 | $4,933,358 |
| BROWN UNIVERSITY | RI | USA | 1 | $241,831 |
| UNIVERSITY OF TENNESSEE HEALTH SCI CTR | TN | USA | 1 | $380,000 |
| UNIVERSITY OF NORTH TEXAS HLTH SCI CTR | TX | USA | 1 | $365,000 |
| UNIVERSITY OF TEXAS MED BR GALVESTON | TX | USA | 1 | $750,254 |
| UT SOUTHWESTERN MEDICAL CENTER | TX | USA | 1 | $405,000 |
| ALTIUS INSTITUTE FOR BIOMEDICAL SCIENCES | WA | USA | 1 | $3,086,945 |
| FRED HUTCHINSON CANCER RESEARCH CENTER | WA | USA | 2 | $5,264,836 |
| SEATTLE CHILDREN’S HOSPITAL | WA | USA | 2 | $1,452,909 |
| UNIVERSITY OF WASHINGTON | WA | USA | 7 | $4,355,021 |
| WASHINGTON STATE UNIVERSITY | WA | USA | 1 | $601,845 |
| UNIVERSITY OF WISCONSIN-MADISON | WI | USA | 3 | $925,660 |
| UNIVERSITY OF WYOMING | WY | USA | 1 | $733,243 |
| CENTRE/AIDS PROGRAMME/RES/SOUTH AFRICA | SO AFR | 1 | $99,360 | |
| UNIVERSIDAD AUTONOMA DE MADRID | SPAIN | 1 | $135,000 | |
| Data downloaded from https://Report.nih.gov on April 7, 2020; Analysis end date: May 1, 2020; Research/Disease Area: Human Fetal Tissue; Fiscal Year Spending Category: FY 2019; *One project shared with Yale University | ||||
Summary and Perspective
This thorough analysis uncovered important facts about the current state of NIH-funded research using human fetal tissue. Of primary importance is that under current policy, NIH is prohibited to fund intramural research using human fetal tissue from elective abortions. However, the NIH is still estimated to spend over $100 million taxpayer dollars in fiscal year 2019 to fund human fetal tissue extramural research. NIH funding for human fetal tissue supports research at 68 different academic organizations throughout the U.S., not including two international organizations in South Africa and Spain.
Extramural research projects that are new or up for competitive renewal after September 25, 2019 and that originally would have proposed use of fetal tissue will now fall into one of the following categories:
- Projects that propose to use human fetal tissue from elective abortions must include a “Human Fetal Tissue Justification” description to ensure that human fetal tissue is utilized for research only when scientifically justifiable. If recommended for potential funding through NIH’s two-level external scientific review process, the project will undergo review by the NIH Human Fetal Tissue Research Ethics Advisory Board. Funding may or may not be recommended based on ethical considerations.
- New projects that exclude human fetal tissue from elective abortions, or competitive renewals that change to no longer use human fetal tissue from elective abortions, may be submitted. These projects may or may not choose to use alternative research materials and models.[2] If recommended for funding through NIH’s two-level external scientific review process, funding is probable.
- Current research projects in the competitive renewal process that use human fetal tissue from elective abortions may be allowed to expire on their project end date, or grantees may extend the final budget period one time for a period of up to 12 months, without additional NIH funds, and without prior approval (No-Cost Extension). Once the extended period ends, the research project may expire or move to categories A or B above to apply for an additional period of funding.
During our analysis, we found significant inadequacies in the way NIH categorizes and reports human fetal tissue research. The NIH does not distinguish between and/or explicitly report human fetal tissue research from miscarriages/stillbirths and elective abortions. Therefore, it is impossible to estimate the fraction of funded projects that use organs and tissues of electively aborted fetuses.
The NIH’s obscuration of the actual number causes uncertainty that can act to minimize perceptions of the significance of the problem. This practice also masks any projects that are successfully using ethically-sourced human fetal tissue from miscarriages/stillbirths. The current NIH policy of not reporting which fetal tissue source is being used (elective abortion or natural death) indicates that NIH leadership also considers there to be no scientific distinction either.
We offer the following recommendations, which would further strengthen NIH funding mechanisms regarding fetal tissue research.
- RECOMMENDATION #1: NIH leadership must make a distinction between elective abortion and miscarriage fetal sources when reporting federal spending. There should be two different NIH Spending Categories for human fetal tissue research: (1) human fetal tissue – elective abortion, and (2) human fetal tissue – non-iatrogenic death. This is no different from current policy that requires the NIH to distinguish between funding for stem cell research using different human cell sources (embryos, hiPSCs, adult stem cells, etc.). Like embryonic stem cell research, there is an ethical distinction to be made with human fetal tissue from elective abortions, and the NIH funding policies and procedures should be consistent with this attitude.
- RECOMMENDATION #2: HHS should prohibit funding of both intramural and extramural research projects that propose to use electively aborted fetal tissue. 100% of all human fetal tissue funding should be directed to research that uses only fetal tissue from the preborn that died a natural death (miscarriage/stillbirth).
Such actions are essential to ensure that every American taxpayer dollar is used to fund research that supports and values the sanctity of every human life and respects the consciences of all scientists, physicians, and patients, without exclusion or exploitation of any group within our society.
Acknowledgement: We thank Michele Rifelj for excellent assistance with research analysis.
Tara Sander Lee, Ph.D., is a Senior Fellow and the Director of Life Sciences at the Charlotte Lozier Institute.
James Sherley, M.D., Ph.D., is an associate scholar at the Charlotte Lozier Institute.
[1] T. Sander Lee, M. B. Feeney, K. M. Schmainda, J. L. Sherley, and D. A. Prentice. Human fetal tissue from elective abortions in research and medicine: science, ethics, and the law. Issues in Law and Medicine, vol. 35, 1:3, 2020
[2] T. Sander Lee, M. B. Feeney, K. M. Schmainda, J. L. Sherley, and D. A. Prentice. Human fetal tissue from elective abortions in research and medicine: science, ethics, and the law. Issues in Law and Medicine, vol. 35, 1:3, 2020