The Treatment of Human Embryos Created through IVF: The U.S. and 15 Selected Countries’ Regulations

(Human embryo being prepared for cryopreservation. Credit: Science Source)
This is Issue 102 of the On Point Series.
Executive Summary
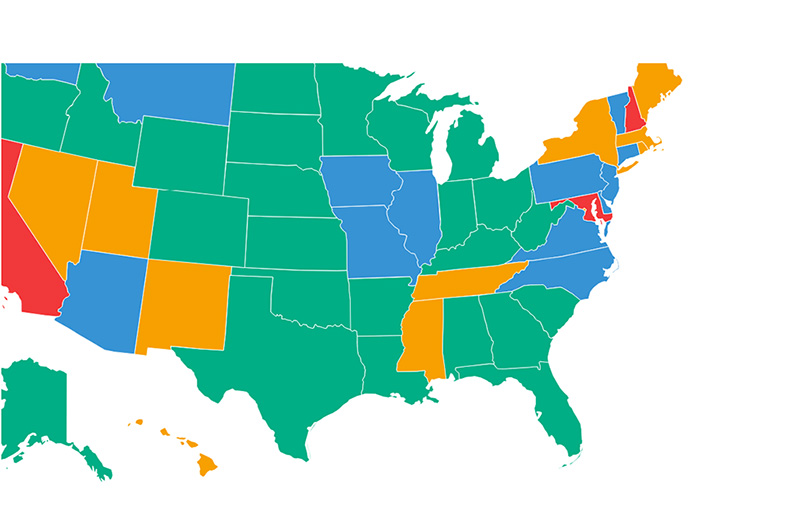
- While countries like Italy, Germany, and Poland have regulated IVF in a way that attempts to protect human life in its earliest stages, the United States has virtually no IVF regulations regarding the treatment of human embryos, allowing for the creation, destruction, and indefinite freezing of countless embryonic human beings.
- Three of the sixteen countries reviewed in this paper (Germany, Poland, and Switzerland) limit the number of human embryos that may be created per cycle, and three limit the number that can be transferred (Germany, Spain, and Sweden).
- Six of the reviewed countries explicitly permit destructive scientific research on human embryos created during IVF, including Belgium, Spain, and the UK, while the United States and six others allow this due to a lack of relevant national regulations.
- Seven of the reviewed countries do not have any relevant federal regulation limiting the time human embryos can be frozen, while another eight have some limit, ranging from five to 55 years.
- Eight of the reviewed countries explicitly permit the donation of human embryos to another person, while the United States and five other countries allow this due to a lack of relevant national regulations.
- Spain and Switzerland explicitly permit parents to choose human embryos for transfer based on their sex (for certain sex-related medical reasons), another six countries prohibit sex selection by default but have medical exceptions, and the United States plus six others allow this due to a lack of relevant national regulations.
This paper examines the present status of in vitro fertilization (IVF) laws, regulations, and guidelines in a sampling of countries with a focus on G12 members.[1] IVF is a process by which a woman’s eggs are harvested and fertilized by sperm outside of the body, creating one or more embryonic human beings which will then be transferred into a uterus.[2] Each attempt at achieving pregnancy through this process is called an IVF “cycle,” and by some estimates, in order to have a high likelihood of a successful pregnancy through IVF, an average of six cycles is needed.[3] The woman and man who contribute the egg and sperm may be a couple or one or both parties may be donors.[4] IVF is also used in surrogacy where embryonic human beings are placed in an unrelated woman’s uterus to gestate on behalf of another person or couple.[5]
Currently, the majority of IVF statutes and ethical guidelines reviewed here permit the creation of multiple embryonic human beings in excess of the number that can or will be implanted per IVF cycle. These excess human embryos are either used for implantation in subsequent cycles, frozen indefinitely, or discarded (in which case the human embryo, a living human being, dies).
Few of the reviewed countries have limits on how many embryonic human beings may be created per IVF cycle, while at least three limit the number of human embryos that may be transferred per cycle. Some European countries, however, permit the creation of only those human embryos who will be implanted in a single cycle. In addition, there are a number of countries that limit the amount of time human embryos may be cryopreserved, typically to between 5-10 years (with extensions possible in some countries). In the likely event that human embryos are not implanted into a uterus before the country’s cryopreservation limit, there are typically three options offered: donation to another person or couple, donation to destructive scientific research (typically just called “research” in statutes), or “disposal” (also, of course, resulting in the death of the embryonic human being). Once the time limit has been reached, some countries automatically implement one of these three options. Also varying by country is whether preimplantation genetic testing (PGT) and/or preimplantation genetic diagnosis (PGD) are permitted.[6] This could allow parents to select or reject human embryos for implantation based upon the results of genetic testing, potentially including the presence or absence of disabilities or information about whether the embryo is male or female (allowing for sex selection).
In most of the reviewed countries, there appears to be very little statutory language protecting the general welfare of embryonic human beings, regulating the creation and/or freezing of so many of them, or considering their ultimate fate.[7]
In the following table, the policies of 16 countries related to IVF are laid out. Though for the most part the information is self-explanatory, a few clarificatory remarks are in order. “No relevant laws” indicates that a country does not nationally regulate IVF by statute at all, and so any way of conducting or failing to conduct the relevant practice (creating excess human embryos, conducting preimplantation genetic testing, etc.) is legal, at least nationally/federally. However, in such countries there may be non-statutory, unenforceable recommendations provided by professional organizations. Relatedly, “Not addressed” indicates that a country does have some statutes regulating the practice of IVF, but that these statutes fail to address the specific practice at issue, which in most cases also implies that this practice can be legally conducted in any way (though, again, there may exist unenforceable professional guidelines). Finally, please note that throughout this paper, the terms “embryonic human being” and “human embryo” are used to describe unborn children in the early stages of development, though many of the countries below use varying terms to describe these children, including “embryo,” “impregnated eggs,” “pre-embryo,” and similar terms. The author does not draw distinctions here between the countries’ terms because the regulations all affect human beings at the earliest stages of development. Following Table 1, further information and sources are provided for each of the 16 countries.
Table 1. Select Countries’ Policies Regarding IVF Regulations**
(Scroll to the right to see full list; table also available in PDF by clicking ‘Print/Download’ above)
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | Country | Limits on # of Human Embryos Created per Cycle | Limits on # of Human Embryos Transferred per Cycle | Cryopreservation Limits | Donation/ Adoption Permitted | Destructive Scientific Research Permitted | Disposal Permitted | PGT/PGD Permitted* | Sex Selection Permitted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | bcook | 21/11/2024 09:42 AM | bcook | 21/11/2024 03:02 PM | Belgium | Not addressed | Not addressed | 5 years, with possibility for extension | Yes | Yes | Yes | No, with some exceptions | No, with some exceptions |
| 3 | bcook | 21/11/2024 09:42 AM | bcook | 21/11/2024 03:08 PM | Canada | Not addressed | Not addressed | Not addressed | Not addressed | Not addressed; permission possibly implied | Not addressed | Not addressed | No, with some exceptions |
| 5 | bcook | 21/11/2024 09:45 AM | bcook | 21/11/2024 02:06 PM | Australia | No relevant laws | No relevant laws; 1-2 embryos recommended by professional guidelines (depending on age) | No relevant laws | No relevant laws; permitted by professional guidelines | No relevant laws; permitted by professional guidelines | No relevant laws; permitted by professional guidelines | No relevant laws; permitted in some cases by professional guidelines | No relevant laws; permitted in some cases by professional guidelines |
| 18 | bcook | 21/11/2024 03:08 PM | bcook | 21/11/2024 03:11 PM | France | Not addressed | Not addressed | 5 years, with possibility for extension | Yes | Not addressed | Yes | No, with some exceptions | Not addressed |
| 19 | bcook | 21/11/2024 03:11 PM | bcook | 21/11/2024 03:20 PM | Germany | As many as will be transferred, but not more than 3 embryos† | 3 embryos | Not addressed | Not addressed | No | No | No, with some exceptions | No, with some exceptions |
| 20 | bcook | 21/11/2024 03:20 PM | bcook | 21/11/2024 03:22 PM | Italy | Only the number of embryos needed for the overall success of the IVF treatment (no limits per cycle) | None | None | No | No | No | Yes, in some cases | Not addressed |
| 22 | bcook | 21/11/2024 03:24 PM | bcook | 21/11/2024 03:24 PM | Japan | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws |
| 24 | bcook | 21/11/2024 03:25 PM | bcook | 21/11/2024 03:25 PM | Mexico | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws |
| 26 | bcook | 21/11/2024 03:28 PM | bcook | 21/11/2024 03:28 PM | Netherlands | Not addressed | Not addressed | Not addressed | Yes | Yes | Not addressed | Not addressed | No, with some exceptions |
| 28 | bcook | 21/11/2024 03:31 PM | bcook | 21/11/2024 03:31 PM | New Zealand | Not addressed | Not addressed; 1-2 embryos recommended by professional guidelines (depending on age) | 10 years, with possibility for extension | Yes | Yes, but must be approved by an ethics committee‡ | Yes | Yes, in some cases | No |
| 30 | bcook | 21/11/2024 03:34 PM | bcook | 21/11/2024 03:34 PM | Poland | Maximum of 6 embryos, with exceptions for more than 6 | Not addressed | 20 years, followed by compulsory donation/ adoption | Yes | No | No | Yes, in some cases | No, with some exceptions |
| 31 | bcook | 21/11/2024 03:34 PM | bcook | 21/11/2024 03:50 PM | Spain | Not addressed | 3 embryos | No uniform limit; assessed on case-by-case basisҰ | Yes, but a maximum number of 6 children may be born from a single donor | Yes | Yes | Yes, in some cases | Yes, in some cases |
| 32 | bcook | 21/11/2024 03:50 PM | bcook | 21/11/2024 03:52 PM | Sweden | Not addressed | 1 embryo; 2 in special cases | 10 years, with possibility for extension | Yes | Yes | Yes | Yes | Not addressed |
| 33 | bcook | 21/11/2024 03:52 PM | bcook | 21/11/2024 03:55 PM | Switzerland | Maximum of 12 embryos | Not addressed | 5 years, with one 5-year extension possible | No | Not addressed | Yes | Yes, in some cases | Yes, in some cases |
| 34 | bcook | 21/11/2024 03:55 PM | bcook | 21/11/2024 03:56 PM | United Kingdom | Not addressed | Not addressed | 55 years, with 10-year renewals | Yes | Yes | Yes | Yes | No, with some exceptions |
| 35 | bcook | 21/11/2024 03:56 PM | bcook | 21/11/2024 03:57 PM | United States | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws | No relevant laws |
| Country | Limits on # of Human Embryos Created per Cycle | Limits on # of Human Embryos Transferred per Cycle | Cryopreservation Limits | Donation/ Adoption Permitted | Destructive Scientific Research Permitted | Disposal Permitted | PGT/PGD Permitted* | Sex Selection Permitted |
** All G12 countries are represented in Table 1. These countries were selected in part due to previous research on their IVF regulations, cited in footnote 1, and in part due to their economic similarity to the United States. Additional countries were included either due to their regulatory considerations of human embryos or their proximity to the United States.
* Preimplantation genetic testing (PGT) and preimplantation genetic diagnosis (PGD)
† Though in practice this “rule of three” might be violated/interpreted loosely (see the “Germany” section below).
‡ See the “New Zealand” section below regarding the ongoing controversy over the in-practice permissibility of embryonic research.
Ұ See the “Spain” section below for details.
Australia
Australia does not currently have national legislation on IVF or Assisted Reproductive Technology (ART) more generally, but there is a movement organized by the Fertility Society of Australia and New Zealand to create “uniform national legislation for ART and IVF providers.”[8] Earlier this year, Australia became the focus of media attention due to a judge’s erroneous claim that the country only allows one human embryo to be created per cycle.[9] In actuality, there is no legal limit on the number of human embryos that may be created per cycle in Australia, but there are professional guidelines that suggest a limit of 1-2 human embryos (depending on the age of the woman) that can or should be transferred to a woman’s uterus per cycle.[10] The country also has no national limit on how long human embryos may remain cryopreserved, but historically the limit was 10 years.[11] Human embryos may be donated to another couple, donated for use in research, or discarded.[12] Preimplantation genetic testing (PGT) is permitted, but only in certain circumstances, by the National Health and Medical Research Council’s Ethical Guidelines.[13] Sex selection is only permitted by the guidelines if “it is to reduce the risk of transmission of a genetic condition, disease or abnormality that would severely limit the quality of life of the person who would be born.”[14]
Belgium
In Belgium, there appear to be no statutes limiting the number of human embryos that can be implanted in a single cycle.[15] Though there does not appear to be a limit on how many human embryos may be created per cycle, couples are required to use all of their cryopreserved human embryos before they may create more.[16] Human embryos who have been cryopreserved may be destroyed, donated, or used for research.[17] They are cryopreserved for five years but parents may shorten or extend the deadline.[18] The law includes a prohibition of “preimplantation genetic diagnosis of a eugenic nature,” and sex selection is prohibited.[19] However, there is an exception for “embryos affected by sex-linked diseases.”[20]
Canada
Canada’s IVF statute has relatively few protections enumerated. The law has no text limiting the number of human embryos created or transferred.[21] It also does not contain any limit on how long human embryos may be cryopreserved.[22] Likewise, there are no provisions allowing or prohibiting the donation or adoption of human embryos. There are no explicit prohibitions or permissions for research, though one provision may be read as implying that such research is permitted.[23] Human embryo disposal and PGT/PGD are equally unaddressed by any text of Canada’s law.[24] However, Canada’s statute explicitly permits sex selection of embryos in cases where the sex is linked to a genetic “disorder or disease.”[25]
France
The law in France does not have any limit either on the number of human embryos created per cycle or on the number transferred into a woman’s uterus.[26] The country’s laws only allow for storage of cryopreserved human embryos for five years.[27] Cryopreserved human embryos are automatically destroyed after five years if the couple does not respond to the clinic and inform them of their current reproductive plans.[28] Neither sex selection nor research are addressed by France’s law. PGT is only permitted in certain cases where doctors certify that the woman or couple have a family history of an incurable, severe genetic disease.[29] Human embryos may be donated to another woman or couple.[30]
Germany
Germany is much more protective of human embryos in its limitations on creation and transfer than other nations. In Germany, only a maximum of three human embryos may be transferred at a time and clinicians may only create as many as will be transferred in a given cycle.[31] There has been some discussion in Germany about whether the so-called “rule of three” is actually implemented in practice[32] and there have been calls to remove it from the law.[33] Germany’s statute does not explicitly regulate cryopreservation, apart from a brief provision stating that only doctors can preserve human embryos, but the statute’s limit on the numbers of eggs that may be fertilized arguably acts as a de facto prohibition on at least most cases of cryopreservation.[34] In practice, however, it seems that cryopreservation may take place in instances where, for whatever reason, not all of the human embryos were transferred.[35] Similarly, the law does not directly address the donation/adoption of human embryos since it prevents excess human embryos from being created. However, it appears that excess human embryos who were not specifically created for the purpose of donation/adoption can be donated/adopted in certain cases.[36] Human embryos may not be donated for research and may not be discarded.[37] Parents may not choose which embryos to implant based on their preference for the child’s sex, unless it is to avoid “Duchenne-type muscular dystrophy or a similarly sex-linked genetic illness.”[38] PGD is prohibited under German law; however, there is an exception based on whether the “genetic pre-disposition” of the woman or man indicates a high likelihood of “serious genetic illness,” which also requires informed consent.[39]
Italy
Italy was originally as protective of human embryos as Germany, but a higher court ruled in 2009 that some of the country’s restrictions on IVF were unconstitutional, so they have been relaxed substantially. Originally, only three human embryos could be created and transferred per cycle under Italy’s law, but following the court ruling, excess human embryos may be created per cycle as long as this is considered necessary for the overall success of the IVF treatment and there is no limit to the number that can be transferred.[40] Any human embryos not implanted are now cryopreserved and there are no limits on how long they may be preserved.[41] Italy does not allow human embryos to be donated to another couple or for research purposes.[42] Human embryos also may not be discarded.[43] Following a 2015 ruling by Italian courts and a case before the European Court of Human Rights, PGD is permitted to avoid genetic diseases in Italy.[44] Sex selection is not directly addressed by Italian law, but may be prohibited in most cases due to a provision banning the selection of embryos “for eugenic purposes.”[45]
Japan
While Japan is a G12 country, the country’s primary law pertaining to IVF does not include any explicit or implicit protections for human embryos and appears to refer to future statutes as yet unenacted.[46]
Mexico
Mexico is included in this chart because of its proximity to the United States and its emergence as a destination for affordable IVF. However, Mexico has no regulations of the IVF procedure, resulting in no protections for unborn children created through IVF.[47]
The Netherlands
The Netherlands’ IVF law does not include explicit limitations on the number of human embryos that may be created or transferred per cycle, nor are there explicit limits to how long they may be cryopreserved.[48] Donation or adoption of human embryos is permitted under the statute,[49] as is donation to research.[50] Destruction of embryos is neither prohibited nor permitted by statute, because it is unaddressed.[51] The law, by omitting to mention it, does not prohibit PGT or PGD[52] but does prohibit sex selection of human embryos unless “there is a risk of a serious sex-linked hereditary condition.”[53]
New Zealand
While New Zealand uses the same professional guidelines that govern IVF in Australia to suggest a limit of 1-2 human embryos (depending on the age of the woman) that can or should be transferred to a woman’s uterus, there is no limit on the number of human embryos that may be created under the country’s laws.[54] New Zealand does have a national 10-year limit on how long human embryos may be preserved.[55] PGD is permitted in New Zealand under government-set guidelines,[56] though parents are not permitted to choose the sex of their child.[57] Human embryos may be donated to another woman or couple[58] and destruction is permitted under the law.[59] Research on human embryos is also permitted in New Zealand so long as an ethics committee gives approval for this research.[60] While New Zealand’s law does permit research, the ethics committee has been criticized for rarely granting approval for research in practice.[61]
Poland
Poland is not a G12 country, but the country maintains significant regulations and has some emphasis on respect for human dignity. The nation generally limits creating human embryos to six per cycle, though there are exceptional cases related to age, disease, and previous lack of successful IVF treatment in which more than six may be created.[62] There is no limit, however, on the number of human embryos that may be transferred. Human embryo preservation is limited to 20 years, but donation to another couple is compulsory after those 20 years.[63] Human embryos may not be donated for research,[64] and those “capable of normal development” may not be destroyed.[65] PGD is permitted under Polish law for “medical indications,” and sex selection is prohibited except in cases of serious sex-linked diseases.[66]
Spain
Spain does not have a statutory limit on the number of human embryos that may be created per cycle, but does have a limit of three who may be transferred.[67] The Spanish statute allows human embryos to be cryopreserved “until the time when the medical officers … consider the recipient does not meet the clinically appropriate requirements for the practice of the assisted reproduction technique.”[68] The law also permits the cryopreservation centers to “use them at their discretion” for disposal, research, or donation/adoption if the couple cannot be contacted for two consecutive two-year renewal periods.[69] Spain also allows donation/adoption of human embryos but has a limit of six children who may be born from a single donor.[70] Human embryos may be donated for research so long as they have not developed beyond 14 days post-fertilization,[71] and the statute states that they may also be disposed of in that it permits “the cessation of their conservation without any other use.”[72] In some cases, Spain permits PGD for “the detection of serious hereditary diseases, of early onset and not susceptible to postnatal curative treatment”[73] and sex-selection.[74]
Sweden
Sweden does not limit the number of human embryos that may be created per cycle but restricts transfer to one human embryo per cycle with two permitted in special cases.[75] Human embryos may be preserved for 10 years (with possibility for extension), and they may be donated to another couple or for research.[76] Sweden also permits the disposal of human embryos.[77] PGD is permitted in Sweden if there is a risk of inherited genetic disease.[78] The issue of sex selection is not addressed.
Switzerland
In Switzerland, couples or individuals seeking IVF may create a maximum of 12 human embryos per cycle,[79] and there are no explicit statutory limits to how many may be transferred in a single cycle.[80] The statute allows human embryos to be cryopreserved for five years with one five-year extension permitted.[81] Donation or adoption of human embryos is not permitted under the law[82] and research is not mentioned in the statutory language, though it appears to be prohibited by implication.[83] Disposal is automatic after five years if an extension is not granted.[84] In some cases, both PGT/PGD and sex selection are permitted under the statute.[85]
United Kingdom
The United Kingdom has by far the longest cryopreservation limit: 55 years with renewal authorized by the parents every 10 years.[86] The country does not have limits on the number of human embryos that may be created or transferred in a given cycle,[87] and permits donation to another couple or for research purposes.[88] PGT is permitted under UK law,[89] but sex selection is not permitted unless relating to genetic disorders.[90] Disposal of human embryos is permitted.[91]
United States
The United States lacks critical IVF regulations regarding the creation and treatment of human embryos, and the few regulations it does have are primarily limited to those pertaining to facility regulations, tissue screening, record-keeping, and reporting,[92] many of which are unenforceable.[93] There are also no federal restrictions on PGT,[94] and the professional guidance provided by the American Society for Reproductive Medicine (ASRM) is unenforceable.[95] Similarly, there are no federal statutes relating to sex selection,[96] or limits on the number of human embryos that may be created or transferred.[97] There are no federal statutes that regulate research, but the ASRM guidelines recommend allowing surplus human embryos to be donated for research where consent has been obtained in advance.[98] Nor are there federal statutes regulating the destruction of human embryos.[99] Louisiana and Georgia, however, both have laws that attempt to protect unborn children during the in vitro process.[100]
Conclusion
The regulatory landscape for IVF in the countries highlighted in this study reflects a diverse range of approaches shaped by varying cultural, ethical, and legal frameworks. Several countries, like Belgium, France, and the UK, tend to place greater legal emphasis on protecting patient autonomy, technological advancements, and IVF success rates than on legal protections for embryonic human beings. In contrast, other nations, in particular Germany, Italy, and Poland, have approached IVF with regulations and restrictions that attempt to protect the value of nascent human life. Finally, there are a few countries, including Mexico and the United States, that have virtually no IVF regulations pertaining to the treatment of human embryos, which, in turn, has allowed the destruction and indefinite freezing of countless embryonic human beings.
Amanda Stirone Mansfield, J.D. is an associate scholar for the Charlotte Lozier Institute.
[1] There has been some prior research on regulations of assisted reproductive technology like IVF in G12 countries. See for ex. Kirsten Riggan, “G12 Country Regulations of Assisted Reproductive Technologies,” Dignitas 16, no. 4 (2009): 6–7, https://cbhd.org/dignitas-articles/g12-country-regulations-of-assisted-reproductive-technologies.
[2] “Assisted Reproductive Technologies,” American Society for Reproductive Medicine, Revised 2018, available at https://www.reproductivefacts.org/news-and-publications/fact-sheets-and-infographics/assisted-reproductive-technologies-booklet/; See also Tara Sander Lee and James Sherley, “Handbook of Nascent Human Beings: A Visual Aid for Understanding the Science and Experimentation,” Charlotte Lozier Institute at https://lozierinstitute.org/handbook-of-nascent-human-beings/ (July 7, 2022).
[3] Andrew DAC Smith et al., “Live-Birth Rate Associated with Repeat in Vitro Fertilisation Treatment Cycles,” JAMA 314, no. 24 (December 22, 2015): 2654–62, https://doi.org/10.1001/jama.2015.17296. However, some fertility specialists argue the number of cycles should be limited to three or four, due to the physical and financial cost of extending cycles to six or more (see “How Many IVF Cycles Should I Plan For?,” Reproductive Resource Center Kansas City IVF, September 19, 2023, https://rrc.com/how-many-ivf-cycles-should-i-plan-for/).
[4] “Assisted Reproductive Technologies,” American Society for Reproductive Medicine.
[5] Ibid.
[6] While there are specific types of pre-implantation genetic testing (PGT), “PGT’ is used in this paper as an umbrella term to refer to genetic testing on human embryos to detect abnormal numbers of chromosomes, structural rearrangement of chromosomal material, and/or specific genetic mutations.
[7] For a discussion of bioethical considerations regarding nascent human beings, please see Tara Sander Lee and James Sherley, “A Handbook of Bioethical Considerations Regarding Nascent Human Beings and Their Cells (Handbook II),” Charlotte Lozier Institute, at https://lozierinstitute.org/a-handbook-of-bioethical-considerations-regarding-nascent-human-beings-and-their-cells-handbook-ii/ (October 26, 2022).
[8] Fertility Society of Australia and New Zealand, “National framework to establish Australia-wide legislation for IVF and ART in development,” (last accessed Oct. 14, 2024), available at https://www.fertilitysociety.com.au/media/australia-wide-legislation-for-ivf-and-art-in-development/.
[9] Alex Polyakov, “Alabama Ruling Frozen Embryos are Equivalent to Living Children has Worrying Implications for IVF,” The Conversation (Feb. 26, 2024) https://theconversation.com/alabama-ruling-frozen-embryos-are-equivalent-to-living-children-has-worrying-implications-for-ivf-224365; James LePage, et al. v. The Center for Reproductive Medicine and Mobile Infirmary Association, No. SC-2022-0515, at 44 (Sup. Ct. of Al. 2024) available at https://cases.justia.com/alabama/supreme-court/2024-sc-2022-0579.pdf?ts=1708115406.
[10] Fertility Society of Australia and New Zealand, Reproductive Technology Accreditation Committee, Code of Practice for Assisted Reproductive Technology Units (rev. Oct. 2021) (Code of Practice), §3.3, https://www.fertilitysociety.com.au/wp-content/uploads/20211124-RTAC-ANZ-COP.pdf.
[11] NHMRC, Ethical Guidelines on Assisted Reproductive Technology 11 (1996), https://perma.cc/DFU4-KKWT.
[12] National Health and Medical Research Council (NHMRC), Ethical Guidelines on the Use of Assisted Reproductive Technology in Clinical Practice and Research (2017, updated 2023) (Ethical Guidelines) ¶ 4.1.3., https://www.nhmrc.gov.au/about-us/publications/art.
[13] Ethical Guidelines ¶¶ 8.15.1, 8.15.2, 8.16.1
[14] Ethical Guidelines ¶ 8.13.1., 8.14.1
[15] Loi relative à la procréation médicalement assistée et à la destination des embryons surnuméraires et des gametes. Available at: https://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=fr&la=F&table_name=loi&cn=2007070632; translated through Google Translate.
[16] Title IV, Chapter 1, Article 9
[17] Title IV, Chapter 1, Article 10
[18] Title IV, Chapter 2, Article 17; Title IV, Chapter 2, Article 18
[19] Title VI, Chapter 2, Article 67
[20] Ibid.
[21] See generally Assisted Human Reproduction Act, Last Amended June 9, 2020, available at https://laws-lois.justice.gc.ca/PDF/A-13.4.pdf, not specifying any limits on creating or transferring embryonic human beings.
[22] See Assisted Human Reproduction Act not mentioning cryopreservation or any limits on how long embryonic human beings may be cryopreserved.
[23] See Assisted Human Reproduction Act Sec. 5(b) implying that embryonic human beings may be made for purposes of research.
[24] See generally Assisted Human Reproduction Act not specifying any limitations or permissions for disposal of embryonic human beings or any limitations or permissions for performing PGT/PGD.
[25] Assisted Reproduction act Sec. 5(e).
[26] Code de la santé publique [Public Health Code], art. L2141-3, https://www.legifrance.gouv.fr/download/pdf/legiOrKali?id=LEGITEXT000006072665.pdf&size=15,2%20Mo&pathToFile=/LEGI/TEXT/00/00/06/07/26/65/LEGITEXT000006072665/LEGITEXT000006072665.pdf&title=Code%20de%20la%20sant%C3%A9%20publique.
[27] Public Health Code, art. L2141-4
[28] Ibid.
[29] Public Health Code, art. L2131-4; L2161-1; L2161-2
[30] Public Health Code, art. L2141-5
[31] Embryonenschutzgesetz [ESchG], Dec. 13, 1990, BGBl. I at 2746, § 1, subsec. 1, nos. 3, 4, as amended, https://www.gesetze-im-internet.de/eschg/ESchG.pdf.
[32] See German Ethics Council, “Embryo Donation, Embryo Adoption and Parental Responsibility.” Opinion Mar. 22, 2016 at pg. 11 referring to the inconsistency in practice of limiting the number of embryos created or transferred to three. Available at https://www.ethikrat.org/fileadmin/Publikationen/Stellungnahmen/englisch/opinion-embryo-donation-embryo-adoption-and-parental-responsibility.pdf.
[33] See generally German Medical Association, “Rule of three, egg donation and embryo donation in focus – Memorandum for a reform of the Embryo Protection Act,” Feb. 14, 2020 available at https://www.wbbaek.de/fileadmin/user_upload/_old-files/downloads/pdf-Ordner/MuE/2020-09-11_Memorandum_DAEB_final.pdf (Translated using Google Translate).
[34] ESchG, § 9, no. 4.; and see ESchG § 1, subsec. 1, nos. 3, 4 limiting the number of embryos that may be created and transferred.
[35] See generally, German Medical Association, “Directive for the removal and transfer of human germ cells or germ cell tissue in the context of assisted reproduction,” Jan 14, 2022 available at https://www.wbbaek.de/fileadmin/user_upload/BAEK/Themen/Medizin_und_Ethik/RiLi-ass-Reproduktion.pdf (Translated using Google Translate).
[36] “Embryo Donation,” Deutsche Referenzzentrum für Ethik in den Biowissenschaften, accessed November 14, 2024, https://www.drze.de/en/research-publications/in-focus/research-with-human-embryonic-stem-cells/modules/embryo-donation.
[37] ESchG, § 1, subsec. 1, no. 2; subsec. 2.; and ESchG, § 2
[38] ESchG § 3
[39] ESchG Sec 3a, subsec. 1, 2
[40] Legge 19 febbraio 2004, n. 40 (Law No. 40), Norme in materia di Procreazione Medicalmente Assistita, art. 14.2., https://www.gazzettaufficiale.it/eli/id/2004/02/24/004G0062/sg; but see Sentenza del 8 maggio 2009, n. 151 (Decision No. 151 of 2009), https://www.gazzettaufficiale.it/eli/id/2009/05/13/009C0344/s1.
[41] Decision No. 151 of 2009, Considerations of Law para 5; Law No. 40, art. 14.1.
[42] Law No. 40, art. 13; see also id. art. 12 prohibiting the use of gametes for purposes other than procreation involving the couple.
[43] Decision No. 151 of 2009, Considerations of Law para. 1; see also Law No. 40 Art. 14.1 prohibiting both cryopreservation and killing (the Italian word “soppresione” can be translated as “suppression” or as “killing” or “eliminating”).
[44] Sentenza del 21 ottobre 2015, n. 229 (Decision No. 229 of 2015) Considerato in diritto, para. 2, https://www.gazzettaufficiale.it/eli/id/2015/11/18/T-150229/s1; Costa and Pavan v. Italy (No. 54270/10), at sec. 3, 71, https://perma.cc/QAZ4-QK78.
[45] Law No. 40 art. 13.3(b)
[46] The relevant law related to IVF is the Act on Assisted Reproductive Technology Offering and the Special Provisions of the Civil Code Related to the Parent-Child Relationship of a Child Born As a Result of the Treatment, available at https://www.japaneselawtranslation.go.jp/en/laws/view/4367/en; see generally Japan Assisted Reproductive Technology Standardization Organization (JISART), Guidelines for in vitro fertilization using donated sperm or eggs, last updated Sept. 21, 2021 (translated using Google Translate), providing guidelines for use of ART without force of law. Available at https://jisart.jp/wp-content/uploads/2024/09/%E7%B2%BE%E5%AD%90%E5%8F%88%E3%81%AF%E5%8D%B5%E5%AD%90%E3%81%AE%E6%8F%90%E4%BE%9B%E3%81%AB%E3%82%88%E3%82%8B%E4%BD%93%E5%A4%96%E5%8F%97%E7%B2%BE%E3%81%AB%E9%96%A2%E3%81%99%E3%82%8BJISART%E3%82%AC%E3%82%A4%E3%83%89%E3%83%A9%E3%82%A4%E3%83%B3%EF%BC%88%E6%94%B9%E5%AE%9A240914%EF%BC%89.docx.pdf.
[47] Alma López et al., “The Need for Regulation in the Practice of Human Assisted Reproduction in Mexico. An Overview of the Regulations in the Rest of the World,” Reproductive Health 18 (November 27, 2021): 241, https://doi.org/10.1186/s12978-021-01293-7.
[48] See generally, Embryo Law, 01-07-2021, available at https://wetten.overheid.nl/BWBR0013797/2021-07-01 showing no enumerated restrictions on creation, transfer, or cryopreservation of embryonic human beings.
[49] Embryo Law Art. 8.1.a
[50] Embryo Law Art. 3, Art. 8.1.b,c. See also Art. 9. implying that prior to automatic destruction, the embryonic human beings may be made available for research.
[51] Embryo Law Art. 7
[52] See generally Embryo Law, showing no enumerated restrictions on PGT/PGD.
[53] Embryo Law Art 26
[54] Fertility Society of Australia and New Zealand, Reproductive Technology Accreditation Committee, Code of Practice for Assisted Reproductive Technology Units (rev. Oct. 2021) § 3.3, https://www.fertilitysociety.com.au/wp-content/uploads/20211124-RTAC-ANZ-COP.pdf.
[55] Human Assisted Reproductive Technology Act 2004 (HART Act) s 10(1) & (4), https://www.legislation.govt.nz/act/public/2004/0092/latest/DLM319313.html.
[56] Human Assisted Reproductive Technology Order (HART Order) 2005, sch 1, pt 1, https://legislation.govt.nz/regulation/public/2005/0181/latest/DLM336019.html; ACART, Guidelines on Preimplantation Genetic Diagnosis with Human Leucocyte Antigen Tissue Typing (Aug. 18, 2014), https://acart.health.govt.nz/assets/Uploads/ACART/Publications/acart-pgd-with-hlag-guidelines-aug2014.pdf.
[57] Human Assisted Reproductive Technology Act 2004 (HART Act), Part 2, s 11, https://www.legislation.govt.nz/act/public/2004/0092/latest/whole.html.
[58] HART Act, Part 3.
[59] HART Order, sch 1, pt 1.
[60] HART Act, Part 2, s 19.
[61] See New Zealand Advisory Committee on Assisted Reproductive Technology, “Guidelines for Research on Gametes and Non-viable Embryos,” Mar. 18, 2021, https://acart.health.govt.nz/publications-and-resources/guidelines-issued/guidelines-for-research-on-gametes-and-non-viable-embryos; D. Gareth Jones, “Where does New Zealand stand on permitting research on human embryos?,” The New Zealand Medical Journal 1 August 2014, Vol 127 No 1399: 74-82, https://pubmed.ncbi.nlm.nih.gov/25145308/; and see “Government called on to define law on embryo research,” University of Auckland, Jun. 22, 2018, https://www.auckland.ac.nz/en/news/2018/06/22/government-called-define-law-embryo-research.html.
[62] Act of June 25, 2015, on Infertility Treatment (Act on Infertility Treatment), effective Nov. 1, 2015, last amended in 2020, art. 9, paras. 2 & 3, https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20150001087/U/D20151087Lj.pdf.
[63] Act on Infertility Treatment, art. 21, para. 3, sec. 1; art. 97, paras 1. & 2.; see also art. 30 describing the process for “donation other than partner donation”; and see art. 36 explaining when embryo donation is permitted.
[64] The relevant statute does not directly address research on embryos, but does so indirectly. See generally art. 19 permitting research on “reproductive cells that were not used in the procedure of medically assisted procreation” but not on embryos; and see art. 25 prohibiting creation of embryos for purposes other than procreation.
[65] Act on Infertility Treatment, art. 23, para. 3; art. 83; but see art. 19 permitting destruction of “reproductive cells that were not used in the procedure of medically assisted procreation.”
[66] Act on Infertility Treatment, art. 3, para. 2; art. 26, para. 2; art. 82.
[67] Law 14/2006, of May 26, on assisted human reproduction techniques, No 126, May 27, 2006, BOE-A-2006-9292, Art 3.2, available at https://www.boe.es/buscar/pdf/2006/BOE-A-2006-9292-consolidado.pdf; See generally Law 14/2006 not including statutory language limiting the number of embryonic human beings to be created.
[68] Law 14/2006 Art. 11.3
[69] Law 14/2007 Art. 11.6
[70] Law 14/2006 Art. 5.1-5.7
[71] Law 14/2006 Art. 11.4.c.; Art. 15.1.b; Art. 16
[72] Law 14/2006 Art. 11.4.d
[73] Law 14/2006 Art. 12.1
[74] Law 14/2006 Art 26.2.c.10 listing “Sex selection … for non-therapeutic purposes or unauthorized therapeutics” as “very serious infractions.”
[75] Senaste version av SOSFS 2009:32 Socialstyrelsens föreskrifter och allmänna råd om användning av vävnader och celler i hälso- och sjukvården och vid klinisk forskning m.m. (SOSFS 2009:32), ch. 4, §15, https://www.socialstyrelsen.se/kunskapsstod-och-regler/regler-och-riktlinjer/foreskrifter-och-allmanna-rad/konsoliderade-foreskrifter/200932-om-anvandning-av-vavnader-och-celler-i-halso–och-sjukvarden-och-vid-klinisk-forskning/.
[76] Lag (2006:351) om genetisk integritet m.m. [Act Amending the Act on Genetic Integrity (SFS 2006:351)] (SFS 2023:40), ch. 5, §1, §3, §4; ch. 7, §3 https://www.riksdagen.se/sv/dokument-och-lagar/dokument/svensk-forfattningssamling/lag-2006351-om-genetisk-integritet-m.m_sfs-2006-351/.
[77] Act on Genetic Integrity ch. 5, § 3.
[78] Act on Genetic Integrity ch. 4, § 2.
[79] Loi fédérale sur la procréation médicalement assistée (LPMA) 810.11, December 18, 1998 Art. 17.1, available at https://www.fedlex.admin.ch/eli/cc/2000/554/fr.
[80] See LPMA excluding any explicit provisions limiting the number of embryonic human beings that may be transferred per cycle.
[81] LPMA Art. 16.2
[82] LPMA Art 4
[83] See generally LPMA not including explicit reference to destructive scientific research. But see LPMA Art. 29 punishing the creation or retention of embryonic human beings for purposes other than bringing about a pregnancy. And see LPMA Art. 30.1 punishing the development of an embryonic human being “beyond the stage corresponding to that of physiological implantation.”
[84] LPMA Art 16.4
[85] LPMA Art. 5
[86] Human Fertilisation and Embryology Act 1990, Ch. 14, §3; Sched. 3, §11C-11D, https://www.legislation.gov.uk/ukpga/1990/37/data.pdf.
[87] Hum. Fertilisation & Embryology Auth., Directions Given Under the Human Fertilisation and Embryology Act 1990 (As Amended): Multiple Births (Oct. 1, 2012), https://portal.hfea.gov.uk/media/1463/2017-04-03-general-direction-0003-version-4-final.pdf.
[88] Human Fertilisation & Embryology Act 1990, §14; “Relevant provisions of the second Directive”, §1.
[89] Human Fertilisation and Embryology Act 1990, sched. 2, §1(d); “Pre-Implantation Genetic Testing for Monogenic Disorders (PGT-M) and Pre-Implantation Genetic Testing for Chromosomal Structural Rearrangements (PGT-SR),” Human Fertilisation & Embryology Authority, accessed October 22, 2024, https://www.hfea.gov.uk/treatments/embryo-testing-and-treatments-for-disease/pre-implantation-genetic-testing-for-monogenic-disorders-pgt-m-and-pre-implantation-genetic-testing-for-chromosomal-structural-rearrangements-pgt-sr/.
[90] Human Fertilisation and Embryology Act 1990, §13, sec. 10; & sched. 2, 1ZB.
[91] Human Fertilisation and Embryology Act 1990, §14, (1)(ca).
[92] Fertility Clinic Success Rate and Certification Act of 1992, Section 2(a) of P.L. 102–493 (42 U.S.C. § 263a-1(a)), available at: https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3146.pdf; See also “Policy Documents: The Fertility Clinic Success Rate and Certification Act,” United States Centers for Disease Control and Prevention (CDC), https://www.cdc.gov/art/nass/policy.html; and American Society for Reproductive Medicine, “Oversight of IVF in The US,” https://www.asrm.org/globalassets/_asrm/advocacy-and-policy/advocacy-resources/oversight-of-ivf-in-the-us.pdf (last accessed Aug. 29, 2024).
[93] See generally Mary Harned, “IVF Industry Regulation in the United States: Changes Are Needed to Protect Embryonic Children and their Families,” Charlotte Lozier Institute, at https://lozierinstitute.org/ivf-industry-regulation-in-the-united-states-changes-are-needed-to-protect-embryonic-children-and-their-families/ (November 13, 2024).
[94] 42 USC 263a – 263a-7, available at https://www.govinfo.gov/content/pkg/USCODE-2011-title42/pdf/USCODE-2011-title42-chap6A-subchapII-partF-subpart2-sec263a.pdf; 42 CFR 493, available at https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-493.
[95] American Society for Reproductive Medicine, “Indications and Management of Preimplantation Genetic Testing for Monogenic Conditions: A Committee Opinion (2023),” https://www.asrm.org/practice-guidance/practice-committee-documents/indications-and-management-of-preimplantation-genetic-testing-for-monogenic-conditions-a-committee-opinion-2023/?_t_tags=siteid%3a01216f06-3dc9-4ac9-96da-555740dd020c%2clanguage%3aen&_t_hit.id=ASRM_Models_Pages_ContentPage/_a715a35a-64f5-473b-8738-76b1b09503eb_en&_t_hit.pos=15.
[96] 42 USC 263a, available at https://www.govinfo.gov/content/pkg/USCODE-2011-title42/pdf/USCODE-2011-title42-chap6A-subchapII-partF-subpart2-sec263a.pdf; 42 CFR 493, available at https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-493.
[97] Ibid.; American Society for Reproductive Medicine, “Guidance on the Limits to the number of Embryos to Transfer: A Committee Opinion (2021),” https://www.asrm.org/practice-guidance/practice-committee-documents/guidance-on-the-limits-to-the-number-of-embryos-to-transfer-a—committee-opinion-2021/?_t_tags=siteid%3a01216f06-3dc9-4ac9-96da-555740dd020c%2clanguage%3aen&_t_hit.id=ASRM_Models_Pages_ContentPage/_34c21982-d331-48c8-b109-effceb6b081c_en&_t_hit.pos=25; See Robert Klitzman, “Deciding how many embryos to transfer: ongoing challenges and dilemmas,” Reprod Biomed Soc Online (2016 Dec.), https://www.sciencedirect.com/science/article/pii/S2405661816300144?via%3Dihub, noting that, “For patients <35 years of age with a favourable prognosis, ‘providers should only transfer a single embryo, and not more than two embryos’ (The Practice Committee of the ASRM and the Practice Committee of the SART, 2013).”
[98] American Society for Reproductive Medicine (ASRM), “Disposition of unclaimed embryos: an Ethics Committee opinion (2021),” https://www.asrm.org/practice-guidance/ethics-opinions/disposition-of-unclaimed-embryos-an-ethics-committee-opinion-2021/.
[99] Ibid.
[100] GA Code § 19-8-41 (2022), available at https://law.justia.com/codes/georgia/2022/title-19/chapter-8/article-2/section-19-8-41/; LA-RS 9 §121-133 (1986), available at https://biotech.law.lsu.edu/cases/la/health/embryo_rs.htm.