Eugene C. Tarne
Senior AnalystEugene C. Tarne is a senior analyst with the Charlotte Lozier Institute. He is also the president of Tarne Communications Inc., a communications and issue advocacy company he founded in 1999. For more than 25 years, beginning in 1989, Mr. Tarne served as a communications and media relations consultant to the United States Conference of Catholic Bishops Secretariat for Pro-Life Activities. In this capacity, he works closely with the Pro-Life Secretariat to develop messages, promote issues and legislation, develop new programs and materials and implement communications strategies designed to educate the public and promote pro-life issues in the public square. These issues include abortion, assisted suicide and euthanasia, and bioethical issues, especially cloning and stem cell research. In 1996, Mr. Tarne helped found the Physicians Ad Hoc Coalition for Truth (PHACT), an organization of doctors and other medical professionals formed to bring the medical facts to bear on the partial-birth abortion debate. Mr. Tarne also served as Communications Director for Do No Harm: The Coalition of Americans for Research Ethics, a coalition of scientists, researchers, bioethicists, medical, academic and other professionals, patient advocates, and concerned individuals, established in 1999, to promote the ethical pursuit of stem cell research and regenerative medicine in general, and to provide accurate information on such research. Mr. Tarne graduated from Georgetown University in 1977 with a B.A. in Theology. He received his M.A. in History of Religions from The George Washington University in 1979. He was offered scholarships to Harvard, the University of Chicago and the University of Pennsylvania to pursue a Ph.D. in South Asian Studies. He attended the University of Chicago and later the University of Pennsylvania, where he completed his Ph.D. studies, except for dissertation.
Research Authored
Human Embryonic Stem Cell Research 25 Years On
Twenty-five years ago, scientists first isolated human embryonic stem cells (hESCs). Shortly after this development, the use of these stem cells in medical research had become a major public policy controversy in the United States.
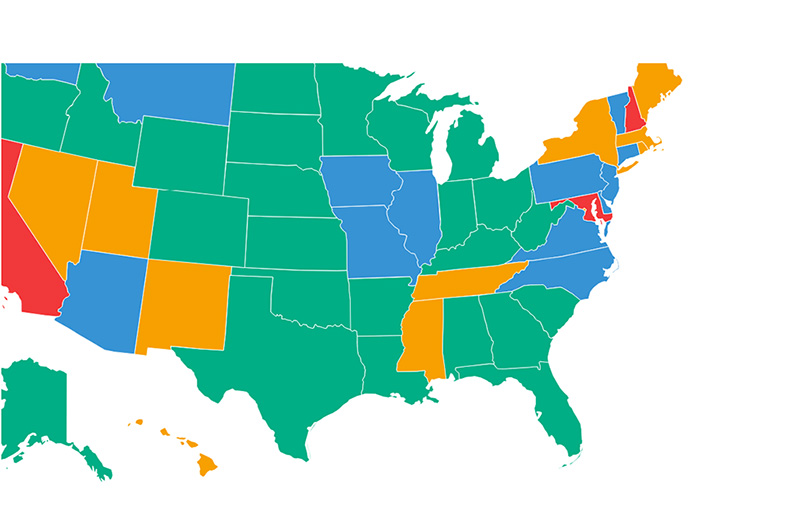
Wiser Investment: Non-Embryonic Stem Cells Receive Millions from Maryland Fund
The state of Maryland is home to the Johns Hopkins University School of Medicine, one of the country’s leading stem cell research programs. Maryland is also one of a handful of states to provide public funds for stem cell research, through its Maryland Stem Cell Research Fund (MSCRF).
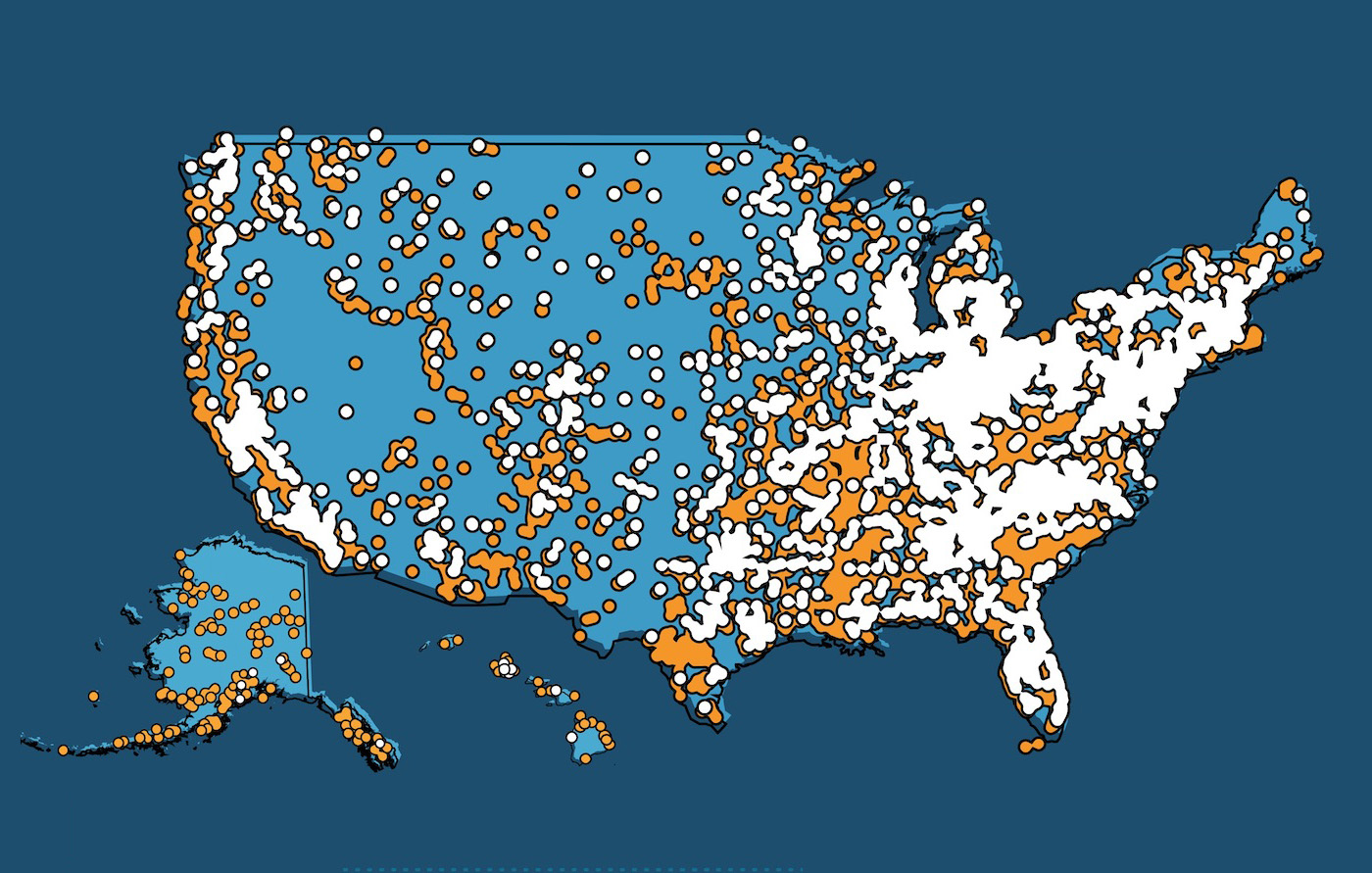
Trends Show More Federal Funds Awarded to Non-Embryonic Stem Cell Research
Soon after then-President Clinton’s National Bioethics Advisory Committee (NBAC) in 1999 issued a report recommending federal funding for human embryonic stem cell research (hESCR) the subject of such funding became one of the nation’s most hotly contested public policy issues. It was the subject of President George W. Bush’s first nationally televised address, and in the ensuing years Congress held a steady stream of hearings on the topic, many of which featured Hollywood celebrities making the case for expansive federal funding of hESCR.
Minnesota Funds Progress through Adult & Induced Pluripotent Stem Cells
The third and most recent round of grants awarded earlier this year for stem cell research by the state of Minnesota tracks a pattern established with the two earlier rounds of grants: a noticeable lack of support for human embryonic stem cell research.
New Non-Embryonic Stem Cell Study Shows Progress with Parkinson’s Disease
Ever since human embryonic stem cells (hESCs) were first successfully grown in the lab in 1998, Parkinson’s Disease has featured prominently as one of the major diseases that such cells would supposedly eliminate.
Netherlands Forcible Euthanasia Case and the Slippery Slope
Proponents of assisted suicide often dismiss “slippery slope” arguments on the grounds that proper safeguards will assure that assisted suicide will not devolve into euthanasia, either voluntary or not. Earlier this year, for example, Hawaii became another of several states to consider
Near Absence of Embryonic Stem Cells in California Clinical Trial Program
In 2013, the California Institute for Regenerative Medicine (CIRM) – the nation’s largest funder of stem cell research outside of the federal government – authorized a new program, the Alpha Stem Cell Clinics Network.
Minnesota’s Funding of Stem Cell Research Echoes Trend toward Ethically Non-Contentious Approaches
In 2014, Minnesota became the most recent of a handful of states that provide state funding for all types of stem cell research, including human embryonic stem cell research (hESCR). The law provides for 10 years of funding with $4.5 million approved for the first year and $4.35 million each year thereafter.
Grants for Stem Cell Research Favor Ethical Approaches
The Maryland Stem Cell Research Fund (MSCRF) has awarded two rounds of grants since the Charlotte Lozier Institute last analyzed the Fund’s pattern of grant making for stem cell research, in the fall of 2013. That study found that since MSCRF first began awarding grants in 2007, its pattern of giving shifted over the years from strongly favoring projects focusing on ethically contentious human embryonic stem cell research (hESCR) to projects focusing on ethically non-contentious adult stem cells and other non-embryonic stem cell research.
iPSCs: A New Gold Standard in Regenerative Medicine?
A recent press release from the National Institutes of Health calls attention to a study, published in Stem Cell Reports, that researchers have “developed a clinical-grade stem cell line, which has the potential to accelerate the advance of new medical applications and cell-based therapies for millions of people suffering from such ailments as Alzheimer's disease, Parkinson's disease, spinal cord injury, diabetes, and muscular dystrophy.”