The Continuing Promise of Non-Embryonic Stem Cells
The California based City of Hope, one of the country’s leading cancer research hospitals, recently sent out birthday greetings to the California Institute for Regenerative Medicine (CIRM), the nation’s leading funder — apart from the federal government – of stem cell research.
“On its 10th birthday, the California Institute for Regenerative Medicine celebrated 10 stem cell therapies that have been approved for clinical trials, including an HIV/AIDS trial at City of Hope,” the message read.
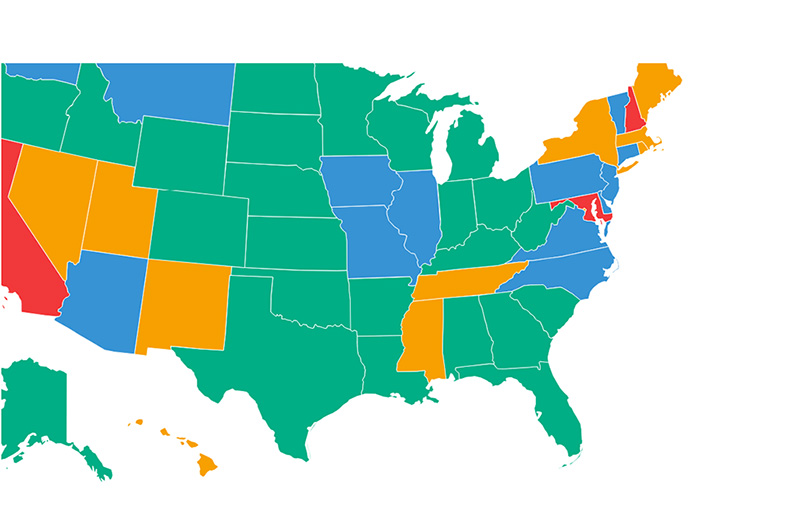
The birthday greeting contained an unintended irony, for while CIRM was approved by California voters a decade ago to give priority funding to human embryonic stem cell research, eight of the 10 approved clinical trials referenced by City of Hope were for research projects using adult and other non-embryonic stem cells.
The fact that 80% of the approved clinical trials based on research projects funded by CIRM are using non-embryonic stem cells is yet further evidence of a trend previously identified on this website: that ethically non-contentious adult and other non-embryonic stem cells are proving far more useful in actually leading to therapeutic benefits for patients than hESCs (human embryonic stem cells), and that this has been reflected in a sustained increase in funding by CIRM for such non-embryonic stem cell research over the years (here and here).
Moreover, research using adult and induced pluripotent stem cells (iPSCs) is providing promising and ethically non-contentious alternatives for treating the conditions in the two clinical trials using hESCs.
One of the hESC trials is for spinal cord injury (SCI). Yet just last month, doctors announced that a man who had been paralyzed from the neck down was able to walk, with assistance, after being treated with his own adult stem cells. (Dozens of other SCI patients have undergone a similar adult stem cell treatment with positive results; see also the research by CLI associate scholar Jean Peduzzi-Nelson.)
The other hESC clinical trial is for retinal degeneration, specifically age-related macular degeneration (AMD).
Here again, non-embryonic stem cells provide an ethically non-contentious alternative to hESCs. Japanese researchers have already conducted an experiment to treat a woman with AMD using retinal tissue developed from her own iPSCs.
Researchers have also discovered adult stem cells in the retinal pigment epithelium (RPE), a cell layer that supports photoreceptors in the eye. In other studies, researchers have used autologous RPE grafts to treat AMD patients, again with promising results.
As referenced before on this website, in 1999, when then-President Clinton’s National Bioethics Advisory Commission (NBAC) first endorsed federal funding for hESC research it nonetheless, in recognition of the ethical problems associated with it, made the pursuit of such research conditional. Harvesting “left-over” IVF embryos for stem cells “is justifiable only if no less morally problematic alternatives are available for advancing the research (at pg. 53).” In other words, given the ethical problems associated with it, hESCR should not be pursued if viable, ethically non-contentious alternatives to it exist. At the time, the NBAC judged that such alternatives did not exist; however, that judgment was provisional and “is a matter that must be revisited continually as science advances.”
That eight of the 10 approved clinical trials based on CIRM-funded research projects are using non-embryonic stem cells is yet more evidence that those alternatives exist. And given the research on AMD and SCI cited above, that 80 percent of approved, non-embryonic, clinical trials could be 100 percent.
Arguably, adult and other non-embryonic stem cell research should no longer be considered the “alternatives” for treating patients.
Gene Tarne is Senior Analyst for Charlotte Lozier Institute.